Spread of antibiotic resistance revives interest in bacteria-killing viruses
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
Bacteria-killing viruses can provide a crucial extra tool in the fight against antibiotic resistance, researchers believe — leading to increased interest in their use, alongside the development of new drugs.
Bacteriophages, or phages, were first discovered and used to treat infection about a century ago. But they were quickly supplanted by antibiotic drugs, which are easier to make and can work against many different bacteria. Estimates of the size of the phage market today range from $42mn to $1.1bn — much smaller than the roughly $43bn market for antibiotics.
However, in recent years, interest in phages has grown as bacteria evolve to become resistant to antibiotic drugs — a development branded a “pandemic” by public health experts and one that caused 1.27mn deaths in 2019.
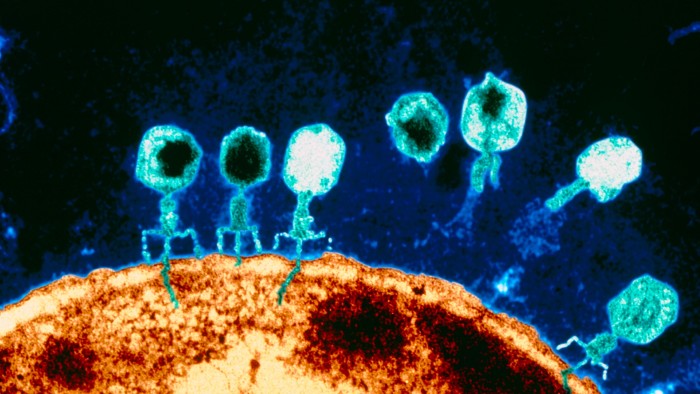
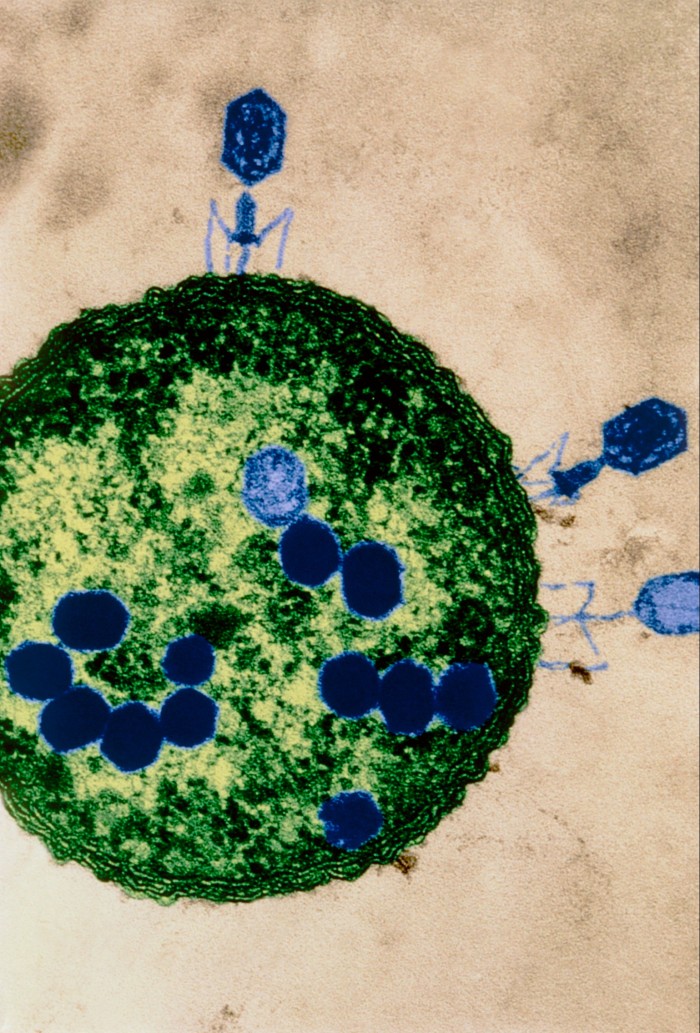
Bacteria and phages are naturally occurring and in constant competition with each other. Every species of bacteria is continually evolving mechanisms to leave its predator phage redundant — prompting the phage to evolve, in turn, to overcome this resistance.
But the great potential for phages as a treatment lies in the fact that there are usually several for each species of bacterium being targeted, and they are specific to it — leaving beneficial bacteria in the body unharmed. While antibiotics can often destroy many types of bacteria by disrupting one of their internal processes, phages attack a specific species of bacteria and can overcome resistance.
“This means . . . there should always be a way to maintain phage effectiveness, for example by alternating treatments,” explains Jason Clark, chief executive of Fixed Phage, a UK supplier. “You can’t do this with antibiotics.” Phages are particularly effective against biofilms — a common feature of severe antibiotic-resistant infections, especially those acquired in hospitals.
Some bacteria and their phages are easier to find than others. “If it were E.coli, I could pop out to my back garden and find some [in soil or pond water] quite easily,” says Clark. One case in 2016 involved an American man who entered a coma and developed multi-organ failure after antibiotics proved ineffective against an infection he acquired on a cruise holiday. Phages derived from sewage were administered to the man and he recovered.
Research also indicates that phage treatments can be effective in ‘last-resort’ cases where antibiotics have failed. A review of 59 clinical studies, published in The Lancet Infectious Diseases last year, found that, of 1,904 patients with chronic and drug-resistant infections treated with phages, 79 per cent showed improvement. Target bacteria were eradicated in 87 per cent of 1,461 cases. Adverse events were reported in only 7 per cent of patients after phage therapy compared with 15 per cent in the control group, and these were “generally mild and resolved after the phage treatment ended”.
More research is needed, though, according to Martha Clokie, professor of microbiology at the University of Leicester and founding editor of the journal Phage. There have been just four clinical trials using predefined combinations of phages to treat infections, all of which failed, but due to technical reasons.
“One failed because they used the wrong phages; one because the phages weren’t stable; and another because the patients got better and went home so there was non-compliance,” she says. “As we go forward, and more people are interested, and people are doing bigger trials, hopefully, the trial design will be improved.”
There are some further barriers to the use of phage treatments in last-resort cases, adds Josh Jones, director of UK Phage Therapy, a non-profit clinical phage supplier. First, the type of bacteria has to be identified, which can take time. Then, if there is a mixture of bacteria in a patient’s infection, phages that can target all of them will have to be found. Finally, the phages can be neutralised by antibodies developed in a patient during a lengthy course of treatment. “The solution is to use another phage,” Jones says. “So there is a way round that.”
But the most significant barrier to widespread use of phages today is regulatory, as current regulations were designed for static antibiotics. There is also a split within the scientific community as to how regulations should change to support phage therapies.
Some argue for a more personalised approach, with bespoke phages for individual patients. This would require completely new regulations and the design of new trials, and is more suited to chronic and drug-resistant infections than to day-to-day infectious diseases.
Others argue that the best approach is to regulate phages more like traditional antibiotics. This means selecting a range of phages in “cocktails” designed to kill all the strains of a species of bacteria. These can then be mass-produced and used as a direct replacement for certain antibiotics.
Clokie believes a mixture of the two will be tried in future: using cocktails for the majority of patients; then utilising the personalised approach if this fails.
“The regulators are often blamed as the gatekeepers,” says Clokie, “but, actually, nobody has come to them with any suggestions. The regulation is not fixed and will move with the science.”
There are signs that the regulatory approach is changing. US company Adaptive Phage Therapeutics is developing a ‘phage bank’ that could be used for personalised treatment, and its chief executive, Greg Merril, says the US Food and Drug Administration, “has recognised there is a significant problem” with the current rules.
He reports that the US regulator’s unit for biologic medicines, which oversees products such as the seasonal flu vaccine, is evaluating APT’s products. “They are experienced in reviewing a product that evolves over time and are leveraging that experience,” Merrill points out.
In the UK, a parliamentary science committee began investigating the barriers to the development and use of phage therapy late last year. It is examining regulation, research, and funding.
Advocates of phages, whether standardised or bespoke, stress that their greatest advantage is that they are not antibiotics — and they should not be evaluated as such.
“It is very difficult to have to constantly measure against antibiotic therapy and have phage therapy fit directly into the box that antibiotics do,” says Francesca Hodges, a microbiologist at Innovate UK, a government agency that provides support for new products and services.
“They are completely different. The whole point of developing phage-based technology is so that antibiotics still work — it is not to replace them completely, but so that we have something else in the toolkit to use alongside antibiotics.”

Comments