Temperature tracker offers sea change for vaccines

Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
Gulf war syndrome was once blamed on the methanol produced when aspartame — an artificial sweetener found in a number of fizzy drinks popular with US troops — is heated past 29.5C. The theory, thought to be behind the spike in medically unexplained illnesses among returning veterans, was soon debunked.
But while it might not matter whether a bottle of Coca-Cola warms up en route from the factory, precision is critical when transporting vaccines.
Most vaccines must be kept between 2C and 8C to remain potent, “but the bigger risk right now is freezing”, says Ben Hubbard, chief executive and cofounder of Parsyl, a software company that monitors how sensitive goods are moved and stored in order to suggest improvements to supply chains.
It was while Hubbard was working for USAID, the US development agency, in sub-Saharan Africa that the idea for Parsyl was born. “We were moving billions of dollars of essential medical supplies and we always struggled with this question around quality,” he says. According to a 2018 review by the journal Vaccine, 37 per cent of vaccines in low-income countries are exposed to temperatures below recommended ranges during storage.

The Denver-based software group places wireless sensors inside refrigerators that carry vaccines from the manufacturer to health facilities in the developing world. Temperature and humidity information is then integrated with other data sets — such as weather and shipping information — to provide two kinds of insights.
“There’s the backward-looking one — what happened to a fridge that registered a drop in temperature, how bad was it, when did it happen?” says Hubbard. “Then there are the forward-looking insights. What can the data we’ve collected on previous shipments and storage locations tell us about the relative risk from a spoilage standpoint . . . or the relative risk of different shipping lanes and so on.”
The group can also simulate potential improvements, which Hubbard says “ultimately makes more vaccines available and saves resources”.
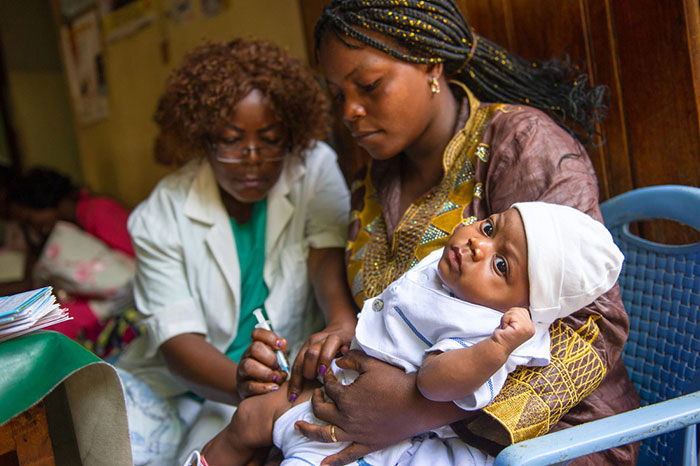
The benefits of expanding global vaccination coverage can be measured in financial terms. A 2019 study by Johns Hopkins University found that across 73 countries, every dollar spent on vaccinating a child saved $21 in healthcare costs, lost wages and lost productivity due to illness or death.
Since 2018, Parsyl has been working with Gavi, a non-profit organisation that raises money to immunise children in poor countries, to shine a light on vaccine — or “cold” — supply chains in Uganda and Senegal. In the past 16 months, Parsyl says it has collected more than 15m data points across both countries, and its work is bearing fruit.
“It used to be that a nurse in a facility in rural Uganda would have to check five fridges, three times a day,” says Shamit Shah, chief executive of Freight in Time, an east African delivery company that partnered with Parsyl in 2018. “Now, all of that information is recorded and sent to the nurse automatically via Bluetooth, meaning he or she has more time to actually do their job.”
“The ministry of health in Senegal is finding [Parsyl’s technology] incredibly important — having that data to hand is giving them a lot more confidence that they are managing their supply chain well,” says Seb Meaney, head of UK strategy in resource mobilisation and innovative finance at Gavi. Parsyl covers 55 per cent of Senegal’s healthcare supply chain system and has been given the green light to expand.

The company’s work has taken on even greater significance given the outbreak of Covid-19, as testing kits that rely on temperature-sensitive reagents are in short supply across Africa.
Also of concern is what effect an outbreak would have on existing vaccination programmes. “The last thing we want is to have an outbreak of a vaccine-preventable disease on top of a Covid-19 outbreak,” says Hubbard. “So to some extent it’s heads down and keep doing what we’re doing.”
“The more we are equipping countries with data to make improvements now, the more robust they’ll be to handle a Covid-19 vaccine when it becomes available, or a malaria vaccine, or an Ebola vaccine.”

Comments