Beyond ‘superbabies’: how Crispr is revolutionising medicine
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
The tail-end of last year brought news that a Chinese scientist had apparently created the world’s first genome-edited babies. It left fellow scientists, and the rest of the world, horrified that such a momentous leap had occurred in secret, with its potential for unintended consequences.
Dr He Jiankui, the clinician behind the breakthrough, is now understood to be under investigation and under guard in China. Little is known about the twin girls, Lulu and Nana, whose genomes he manipulated.
This is the public face of genome editing or, as it is sometimes called, gene editing: a technology capable of creating “superbabies” with optimised DNA, free from disease and tweaked for perfection.
But these are not the applications causing the most excitement in the lab. Instead, the precision offered by tools such as Crispr-Cas9 is revolutionising diagnostics, drug discovery and the treatment of single-gene diseases.
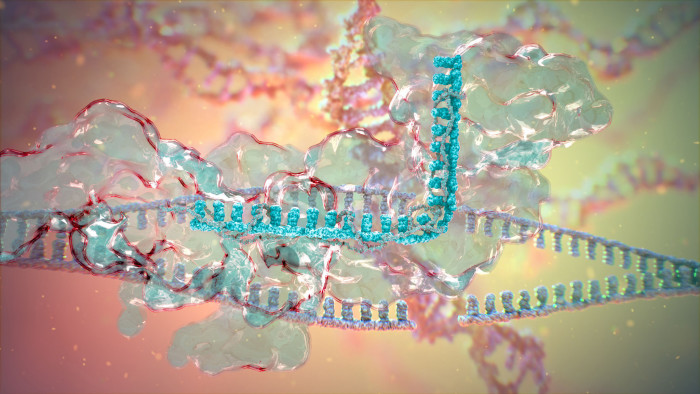
Crispr-Cas9, often shortened to Crispr, is the best-known gene-editing technology. It is a chunk of bacterial genetic code that behaves like a sat-nav, homing in on a specific location in a genome; Cas9 is a cutting enzyme that works like molecular scissors, snipping out portions of DNA. Cellular repair mechanisms then kick in, which can disable, mutate or fix the gene.
“The targets we’re finding with Crispr-Cas9 are going to guide the drugs coming out in the 2020s,” Jon Moore, chief technology officer for Horizon Discovery, a UK biotech company, told Nature.
The technology is simple to use and highly precise. By pairing Crispr-Cas9 with a guide molecule, scientists can edit one spot in the genome with a low chance of unintentional changes elsewhere.
There are thought to be around 10,000 disorders resulting from a single mutation, such as cystic fibrosis. “Many scientists consider genome editing to have great potential for dealing with inherited genetic disorders,” says Professor Robin Lovell-Badge, a developmental biologist and geneticist at the Francis Crick Institute in London.
Genome editing is also proving useful for “knockout screening”, a popular approach in R&D. By snipping out a gene and seeing what functions are affected or which disorders appear, drug targets can be identified. It is also a clever way of facing down drug resistance: researchers can knock genes out of cells, flood the cells with drugs, and then see whether those cells become more sensitive to treatment.
By targeting the proteins made by genes involved with resistance, chemotherapy can be made more effective for conditions such as pancreatic cancer.
Clues can be gleaned about the direction of R&D by seeing where founders in the field have placed their bets. Several scientists are popularly credited with developing Crispr from 2012 onwards and all have started companies to commercialise various applications. The flurry of activity has also triggered a fierce battle over patents.
Biologists Jennifer Doudna and Emmanuelle Charpentier are the doyennes of Crispr: their landmark paper in 2012 showed that the bacterial immune system could be repurposed for genome-editing. They have both picked up prestigious medals and honours, including the $3m Breakthrough Prize, an international award for scientific advances from a coterie of Silicon Valley billionaires, including Mark Zuckerberg.

Last year Professor Doudna, from the University of California, Berkeley, founded Mammoth Biosciences with a focus on diagnostics. This approach uses Crispr as a detector for cheap, simple and speedy diagnostics for disease outbreaks or for use in hospitals.
Professor Charpentier, now a director at the Max Planck Institute for Infection Biology in Berlin, co-founded a different company, Crispr Therapeutics, to focus on treatments for single-gene disorders. Together with Vertex Pharmaceuticals, Crispr Therapeutics earned the green light for trials of a gene therapy codenamed CTX001, which targets sickle cell disease and beta-thalassaemia, serious inherited blood disorders.
The therapy involves taking the patient’s own stem cells, editing them outside the body to produce high levels of foetal haemoglobin, then reintroducing the engineered cells back into the body. The idea, in the case of beta-thalassaemia, is to cut the need for expensive blood transfusions.
Inherited eye diseases are also thought to offer good prospects. Editas Medicine, co-founded by Massachusetts Institute of Technology’s Feng Zhang, another pivotal figure in Crispr development, is focusing on LCA10, a disease produced by an inherited mutation that causes severe vision loss or blindness at birth.
What we don’t want is a knee-jerk reaction from regulators
Prof lovell-badge
In November, Editas won approval from the US Food and Drug Administration to begin enrolling for trials of LCA10 patients, where the gene-editing is done inside the body rather than in cells that have been extracted first, treated and then returned. The pressing question is whether in-vivo (in body) editing will have unintended genetic consequences. However, if successful, it should be cheaper than ex-vivo (out of body) preparations, which require treatments to be tailored separately for each patient. All the therapies being investigated involve editing somatic (non-reproductive) cells. This means that, unlike in the case of Lulu and Nana, alterations will not cascade into future generations.
This brings us to the big unknown in genome-editing: China. The country is keen to lead — there are reportedly several clinical trials, many with cancer patients, under way already — but it risks becoming an international pariah for lax monitoring. Some trial organisers have already admitted losing track of participants, which means long-term side effects will not be catalogued. There are also reports of patient deaths, although these may be due to aggressive tumours.
Professor Lovell-Badge hopes that last year’s bombshell will focus minds without jeopardising a hugely promising technology: “There’s a lot of good work in China but the country needs to ensure that regulatory systems and oversight are robust enough to prevent misuse. What we don’t want is a knee-jerk reaction from regulators to ban genome editing. A ban won’t stop characters like He Jainkui, who knowingly broke guidelines, but it also won’t help progress.”
Comments