Russia vaccine thrusts little-known state research unit into spotlight | Free to read

Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
When Russia announced this week that it had granted the world’s first regulatory approval for a Covid-19 vaccine after just two months of human trials, many in the global pharmaceutical community were aghast at how quickly Moscow had deemed it safe to use.
Russia’s drug companies were comparatively small, little was known of the institute credited with the breakthrough and key steps needed to approve a shot for use had not been completed, critics said. But the man behind the vaccine insists instead that the success has been two decades in the making.
“[The speed] is not surprising if you understand the science behind it,” said Alexander Gintsburg, director of the state-run Gamaleya Research Institute of Epidemiology and Microbiology, which says it has worked on adenoviral vaccines like its Covid-19 shot since the 1980s.
“In the absence of global health threats in recent decades, vaccine research has been on the fringes of the global pharmaceutical industry while Russian labs continued their research,” he told the Financial Times. “We are proud of the legacy of Russian science, which allowed us to develop the Covid-19 vaccine very quickly.”
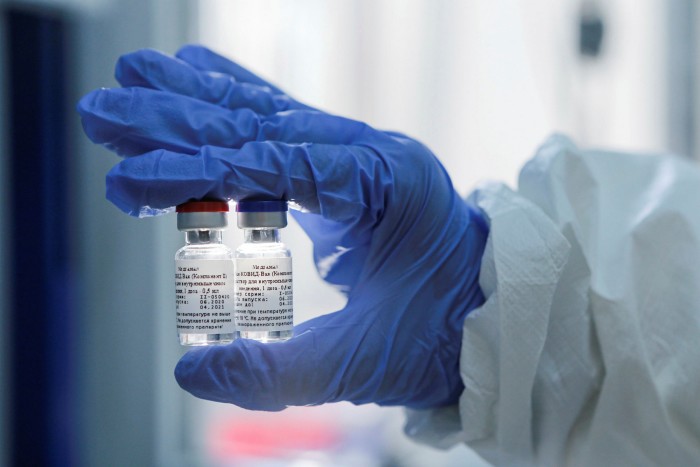
Until this week, few outside Russia knew of the institute, named after the scientist Nikolay Gamaleya who worked under French biologist Louis Pasteur in 1886 and was instrumental in vaccinating Red Army soldiers against smallpox. Even fewer thought it would beat British and US rivals backed by multibillion-dollar pharma giants to begin public vaccinations. On the orders of President Vladimir Putin, frontline medical staff will start receiving injections of Sputnik V, as the vaccine has been named, in the next few weeks.


But the Gamaleya Institute has not necessarily been faster than other vaccine developers. Mr Putin’s decision saw Sputnik V leapfrog rival vaccines in development by approving it before completing so-called Phase 3 trials to check for safety and long-term effectiveness, which can involve thousands of volunteers and take years. That process only began in Russia this week.
Mr Gintsburg pushed back against any suggestion of heightened risk. He and a small group of colleagues at the institute first trialled the vaccine on themselves back in March, he told state television this week.
“After five months, our immunity is good, it corresponds to a significantly high level of antibodies, which guarantees protection against any dose of Covid-19,” he said. “So, at the institute we can afford to be at some liberty to be completely without face masks.”
Defending the decision to roll out public vaccinations of what is still in effect an experimental drug, the Russian government and other supporters have pointed to the successful Ebola vaccine developed by Mr Gintsburg and his colleagues in 2015, the Gamaleya Institute’s most well-known previous success. Sputnik V is based on the same technology.
“For us, the registration of the vaccine was not a surprise . . . it did not just appear from nowhere; it came about as a result of lengthy scientific work,” said Nikolay Bespalov, head of business development at RNC Pharma, a Russian industry consultancy.
“The fact that Russian scientists can develop, and the Russian pharmaceutical industry can produce, drugs requiring intensive scientific research has long been no secret,” Mr Bespalov added. “In Russia, there is a background of serious scientific schooling, a plethora of scientists and organisations to back them up and significant historical experience.”
But while the Soviet Union boasted a high standard of scientific research, the large drop in funding and exodus of talent caused by its collapse have left Russia as a pharmaceutical minnow in global terms.
Last year imports accounted for roughly 60 per cent of Russia’s drug market, according to government statistics. Binnopharm, the Russian company contracted to manufacture the Gamaleya Institute’s vaccine, only has capacity to produce 1.5m doses a year. In comparison, the British government on Friday struck an agreement with US drugmakers Johnson & Johnson and Novavax that takes its total number of ordered vaccine doses to at least 340m.

“Russia does not have any leading pharma companies. Most of its pharma research takes place in state institutions and less information comes out about it than from western or Chinese researchers,” said Rasmus Bech Hansen, chief executive of Airfinity, a London-based science analytics company.
In contrast to vaccines being developed in the UK and the US, where the government’s role has been to provide financial grants and advance purchase orders to private companies and researchers, Russia’s vaccine development has been wholly state-managed.
The Gamaleya Institute is government-controlled, the vaccine was funded by the sovereign wealth fund and an employee of the ministry of defence is named as an author of the vaccine. Senior government officials were given preapproval injections.
Latest coronavirus news
Follow FT's live coverage and analysis of the global pandemic and the rapidly evolving economic crisis here.
Danny Altmann, professor of immunology at Imperial College London, said Russia has good biomedical scientists, but that the combination of nationalism, the lack of transparency and even the vaccine’s name seemed to hark back to the Soviet era. “What we don’t want now is a cold war-style space race in Covid vaccines,” he said.
The World Health Organization told the FT that it could not comment on the vaccine as it had not yet seen the data behind it.
Moscow did not officially approach the WHO about pre-qualifying its vaccine, a necessary step for international use, until Thursday — two days after Mr Putin’s announcement — when its Geneva-based ambassador sent a letter to Tedros Ghebreyesus, the organisation’s head. “We are looking forward to receiving more details and data, as stated in [the] letter,” said Emer Cooke, the WHO’s director of regulation and pre-qualification.
The Gamaleya Institute has said that it plans to submit the results of its clinical trials “to a top-rated journal in two weeks”, but that it was traditional in Russia to first have such data approved privately by experts before public release.
“Russian science has been on the map for centuries,” said Mr Gintsburg. “For us it is important to protect our people from the pandemic and to be able to offer this technology to the world.”

Comments